On Wednesday, August 17, the Food and Drug Administration approved Zelboraf, a drug for BRAF V600E mutation-positive metastatic melanoma. Along with skin cancer drug, FDA approved Companion Diagnostic, a mutation diagnostic test.
“Today’s approval of Zelboraf and the (companion) test is a great example of how companion diagnostics can be developed and used to ensure patients are exposed to highly effective, more personalized therapies in a safe manner,” said Alberto Gutierrez, head of the FDA’s Office of In Vitro Diagnostic Device Evaluation and Safety.

Zelboraf may help metastatic melanoma patient to live longer
Zelboraf and its Companion Diagnostic are co-developed by Roche (Genentech unit) and Plexxikon, Daiichi Sankyo Group member. Roche and Daiichii will promote this medicine in United States. Zelboraf will be available within two weeks and will be distributed through specialty pharmacies (mail-order pharmacies).
Zelboraf (vemurafenib) is a targeted medicine for inoperable or metastatic melanoma BRAF V600E mutation-positive.
“As the first personalized medicine approved for the treatment of patients with BRAFV600E mutation-positive melanoma, Zelboraf represents a significant new treatment option for these patients suffering from inoperable or metastatic melanoma. We are grateful for the clinical trial participants, clinical investigators, collaborators and dedicated employees, who have all contributed to this important advancement in cancer treatment,” said K. Peter Hirth, Ph.D., chief executive officer of Plexxikon.
The cobas 4800 BRAF V600 Mutation Test identifies tumors that carry the BRAF V600E mutation. In this way the doctors will know what person with inoperable or metastatic melanoma can be treated with Zelboraf. This personalized drug has no known beneficial effect on other skin cancers, thus it is important to have a diagnostic test before taking Zelboraf pills.
“The cobas BRAF Mutation Test has improved sensitivity, accuracy and speed compared to other commonly used, unapproved detection methods,” said Paul Brown, head of Roche Molecular Systems.
Studies have shown that Zelboraf increases the survival rate in patients with BRAF V600E mutation-positive melanoma.
According to the American Cancer Society, melanoma, a type of skin cancer, is one of the deadliest cancers, with a five-year survival rate of 15 percent for people with advanced melanoma. It is estimated that BRAF mutations appear in about half of all cases of melanoma and may lead to uncontrolled cell growth.
More information about ongoing Zelboraf studies is available at www.roche-trials.com or www.clinicaltrialsregister.eu (in the EU) or www.clinicaltrials.gov (in the US).
Zelboraf might have side effects. The patients should talk to their doctors about their conditions prior the medication and carefully observe the changes that might appear during the treatment.
“Patients should call their doctor for medical advice about any side effects. Patients or their caregivers are encouraged to report negative side effects of prescription drugs to the FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch. They may also report side effects to Genentech at 1-888-835-2555.
Patients should read the Zelboraf full Prescribing Information and Medication Guide for additional important safety information at www.zelboraf.com.”
In March, the FDA approved Bristol-Myers Squibb’s Yervoy (ipilimumab), an intravenous medicine for patient with advanced melanoma. Roche and Bristol have agreed to collaborate in order to find out if these two medicines are safe and effective if taken together. Roche submitted drug applications for Zelboraf in the EU, Switzerland, Australia, New Zealand, Brazil, India, Mexico and Canada.
[googlead tip=”patrat_mediu” aliniat=”stanga”]Swedish researchers from Lund University have identified a new treatment method for the advanced prostate cancer that may attack and destroy prostate cancer stem cells.
The current common prostate cancer treatments like radiation, hormone therapy and even surgery are ineffective in completely eradicating the tumor.
However, the Swedish team is currently working on developing a new therapy that attacks prostate tumors at the root. This is great news and gives hope to those men who have received positive PSA (Prostate-Specific Antigen) tests for prostate cancer.
Researchers from Lund University have identified a method that may attack and destroy prostate cancer stem cells. These cells can be extremely resistant to most common treatments and if they are not completely destroyed following radiation or surgery, they can cause the cancer to return.[googlead tip=”patrat_mic” aliniat=”dreapta”]

Researchers from Lund University have identified a method that may attack and destroy prostate cancer stem cells
According to the researchers’ statement post on the university website, the new therapy works by targeting a specific protein in prostate cancer stem cells. They found that the STAT3 protein is integral to the ability of stem cells to grow and regenerate. They also noted that the natural compound galiellalactone inhibits the activity of STAT3.
Prostate cancer that has become resistant to hormone treatment and that does not respond to radiation or chemotherapy requires new methods of treatment. By attacking stem cell-like cells in prostate cancer, researchers at Lund University are working on a project to develop a new treatment option.
A successful interdisciplinary project is underway between two research groups, in which senior researcher Rebecka Hellsten and Professor Anders Bjartell at the Faculty of Medicine’s division for Urological Cancer Research, Skåne University Hospital in Malmö, and Professor Olov Sterner and Assistant Professor Martin Johansson at the Lund University division of Organic Chemistry recently published their latest research findings in the scientific online journal PLoS ONE.
“Prostatic tumors are thought to consist only of about 0.1 per cent cancer stem cells, but if you are not successful in eradicating that tumor cell population, there is a risk of subsequent uncontrolled growth of the tumor. The cancer stem cells are often unresponsive to both hormonal treatment and to chemotherapy, so it is essential to develop a direct treatment towards all types of cancer cells”, says Anders Bjartell.
Exploring the tumor biology of prostate cancer, the research group have now observed that the protein STAT3 is active in the stem cell-like cells. In their previous studies, they have proven that the natural compound galiellalactone affects STAT3 and has inhibitory effects on the growth of prostate cancer.
Through the development of new specific STAT3-inhibitors with galiellalactone as a model, the researchers hope to develop targeted therapies that attack the stem cell-like cancer cells in prostate cancer and prevent the tumor from growing and spreading.
[googlead tip=”lista_medie” aliniat=”stanga”]The discovery is still in its very early stages. However, the researchers said they believe using this knowledge to design new pharmaceuticals could significantly improve existing prostate cancer treatments. Any treatment derived from this method would still be a number of years away.
[googlead tip=”patrat_mediu” aliniat=”stanga”]A new treatment for leukemia had amazing results, surprising even the researchers who designed it. The new treatment has eradicated the cancer cells present in the first three patients tested bodies.
Early results of a clinical trial showed that genetically engineered T cells eradicate leukemia cells and thrive.
Scientists from the University of Pennsylvania have genetically engineered patients’ T cells — a type of white blood cell — to attack cancer cells in advanced cases of a common type of leukemia.
The first two of three patients studied, who received the innovative treatment, have been cancer-free for more than one year. In the case of the third patient, over 70% of cancer cells were removed, according to the researchers.
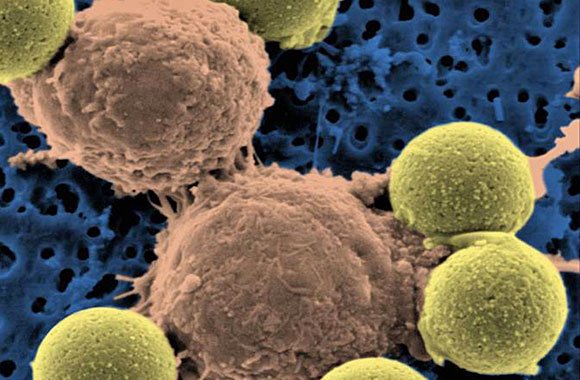
"Microscopic image showing two T cells binding to beads, depicted in yellow, that cause the cells to divide. After the beads are removed, the T cells are infused into cancer patients." (Dr. Carl June / Pennsylvania Medicine)
“In just three weeks, tumors were destroyed, the effect being more violent than we ever have imagined,” said Dr. Carl June, one of the researchers involved in the study.
“Each cell can destroyed thousands of cancer cells,” said June, “each patient have been removed tumors from at least 900 grams.”
“A huge accomplishment”
[googlead tip=”vertical_mare” aliniat=”dreapta”] “This is a huge accomplishment — huge,” said Dr. Lee M. Nadler, dean for clinical and translational research at Harvard Medical School, who discovered the molecule on cancer cells that the Pennsylvania team’s engineered T cells target.
Innovative treatment is using patients’ own T cells, which are extracted from body cells and then genetically modified to attack cancer cells and to multiply and then reintroduced into patients’ blood.
Findings of the trial were reported Wednesday in the New England Journal of Medicine and Science Translational Medicine.
According to LA Times report, for building the cancer-attacking cells, the researchers modified a virus to carry instructions for making a molecule that binds with leukemia cells and directs T cells to kill them. Then they drew blood from three patients who suffered from chronic lymphocytic leukemia and infected their T cells with the virus.
When they infused the blood back into the patients, the engineered T cells successfully eradicated cancer cells, multiplied to more than 1,000 times in number and survived for months. They even produced dormant “memory” T cells that might spring back to life if the cancer was to return.
On average, the team calculated, each engineered T cell eradicated at least 1,000 cancer cells.
Side effects included loss of normal B cells, another type of white blood cell, which are also attacked by the modified T cells, and tumor lysis syndrome, a complication caused by the breakdown of cancer cells.
“We knew [the therapy] could be very potent,” said Dr. David Porter, director of the blood and marrow transplantation program at the Hospital of the University of Pennsylvania in Philadelphia and a coauthor of both papers, which were published in the New England Journal of Medicine and Science Translational Medicine.
“But I don’t think we expected it to be this dramatic on this go-around.”
Bone marrow transplants from healthy donors have been effective in fighting some cancers, including chronic lymphocytic leukemia, but the treatment can cause side effects such as infections, liver and lung damage, even death.
“1/5 of bone marrow transplant recipients may die of complications unrelated to their cancer,” Porter said.
Researchers have been working for many years to develop cancer treatments that leverage a patient’s immune system to kill tumors with much greater precision.
Specialists not involved in the trial said the new discovery is very important because it suggested that T cells could be adapted to destroy a range of cancer cells, including ones of the blood, breast or colon
“It is kind of a holy grail,” said Dr. Gary Schiller, a researcher from UCLA’s Jonsson Comprehensive Cancer Center who was not involved in the trial.
“It would be great if this could be applied to acute leukemia, where there is a terrible unmet medical need,” UCLA’s Schiller said.
Dr. David Porter added:
“Previously efforts to replace risky bone marrow transplants with such engineered T cells proved disappointing because the cells were unable to multiply or survive in patients.”
“This time, the T cells were more robust because the team added extra instructions to their virus to help the T cells multiply, survive and attack more aggressively.”
“About 15,000 patients are diagnosed with chronic lymphocytic leukemia every year. Many can live with the disease for years. Bone marrow transplants are the only treatment that eradicates the cancer.”
[googlead tip=”lista_mare” aliniat=”stanga”]Dr. David Porter cautioned that these were preliminary results and the scientists plan to continue the trial, treating more patients and following them over longer periods.
“The researchers also would like to expand the work to other tumor types and diseases,” Porter said.
The hope, scientists said, is that the method would work for cancers that can kill more ruthlessly and rapidly.
[googlead tip=”patrat_mare” aliniat=”stanga”]
Progressive telomere shortening characterizes familial breast cancer patients
Telomere chromosome
Telomeres, the complex structures that protect the end of chromosomes, of peripheral blood cells are significantly shorter in patients with familial breast cancer than in the general population. Results of the study carried out by the Human Genetics Group of the Spanish National Cancer Research Centre (CNIO), led by Javier Benitez, to be published in open-access journal PLoS Genetics on July 28th, reflect that familial, but not sporadic, breast cancer cases are characterized by shorter telomeres. Importantly, they also provide evidence for telomere shortening as a mechanism of genetic anticipation, the successively earlier onset of cancer down generations.
Mutations in two DNA repair genes, BRCA1 and BRCA2, characterize some, but not all, instances of hereditary breast cancer. Non-BRCA1/2 breast cancer families are heterogeneous, suggesting the existence of other genes conferring susceptibility. The group has investigated the role of telomere length in hereditary breast cancer based on previous information suggesting, first, that short telomeres and subsequent genomic instability contribute to malignant transformation; second, that genetic anticipation occurs in breast cancer families and, third, that telomere shortening is associated with anticipation in other genetic diseases. [googlead tip=”vertical_mare” aliniat=”stanga”]
By analyzing telomere length differences between mothers and daughters from breast cancer families, the authors demonstrated that genetic anticipation is associated with a decrease in telomere length in affected daughters relative to their mothers.
The results allowed the authors not only to conclude that women carrying BRCA1/2 mutation have chromosomes with short telomeres, but also to describe for the first time that genetic anticipation in breast cancer could be explained by telomere shortening. In addition, the study expands the field of research concerning genetic predisposition to breast cancer to include genes involved in telomere maintenance. The significance of generational changes in telomere length has interesting potential clinical applications in the management of familial breast cancer, and could be extended to other hereditary cancer syndromes.
###
FINANCIAL DISCLOSURE: This work was supported by Asociación Española Contra el Cancer (AECC) and Spanish Fondo de Investigaciones Sanitarias (grant numbers FISPI081298 and FIS-PI081120). The CIBER de Enfermedades Raras is an initiative of the ISCIII. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
COMPETING INTERESTS: The authors have declared that no competing interests exist.
CITATION: Martinez-Delgado B, Yanowsky K, Inglada-Perez L, Domingo S, Urioste M, et al. (2011) Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer. PLoS Genet 7(7): e1002182. doi:10.1371/journal.pgen.1002182
Contact:
Dr. Beatriz Martinez-Delgado and Dr. Javier Benitez
Spanish National Cancer Research Centre (CNIO)
Human Genetics
Melchor Fernandez Almagro 3
Madrid 28029
SPAIN
[email protected]
[email protected]
Disclaimer
This press release refers to an upcoming article in PLoS Genetics. The release is provided by journal staff, or by the article authors and/or their institutions. Any opinions expressed in this release or article are the personal views of the journal staff and/or article contributors, and do not necessarily represent the views or policies of PLoS. PLoS expressly disclaims any and all warranties and liability in connection with the information found in the releases and articles and your use of such information.
About PLoS Genetics
[googlead tip=”lista_medie” aliniat=”stanga”]PLoS Genetics (http://www.plosgenetics.org) reflects the full breadth and interdisciplinary nature of genetics and genomics research by publishing outstanding original contributions in all areas of biology. All works published in PLoS Genetics are open access. Everything is immediately and freely available online throughout the world subject only to the condition that the original authorship and source are properly attributed. Copyright is retained by the authors. The Public Library of Science uses the Creative Commons Attribution License. [googlead tip=”lista_medie” aliniat=”centrat”]
About the Public Library of Science
The Public Library of Science (PLoS) is a non-profit organization of scientists and physicians committed to making the world’s scientific and medical literature a freely available public resource. For more information, visit http://www.plos.org.
Potential breakthrough in cancer research: a new treatment for leukemia had amazing results.




