Home Tags Posts tagged with "hiv aids"
hiv aids
The new World Health Organization guidelines for HIV treatment could see millions more people in developing countries getting life-saving medicine.
The WHO is recommending that patients start taking medication at a much earlier stage of the disease.
The WHO says the guidelines, which are being launched at an international Aids conference in Kuala Lumpur, could help avert an extra 3 million AIDS deaths by 2025.
The charity MSF welcomed the move – but said extra investment would be needed.
A single pill combining three drugs will be given to people who are HIV positive much earlier, while their immune systems are still strong. Algeria, Argentina and Brazil are already doing this.
Not everybody who needs the medicine currently receives it, although big strides have been made in recent years in widening access to HIV treatment.
The WHO says these guidelines represent a “major shift” in policy, and will result in the number of people in developing countries who are eligible for drug treatment rising from 16 million to 26 million, or 80% of the total who are thought to have HIV.
It is thought the guidelines will add 10% to the $23 billion overall cost of treating HIV/AIDS in developing countries.

WHO recommends HIV/AIDS patients start taking medication at a much earlier stage of the disease
WHO believes global donors and the affected countries themselves will be convinced that the idea is cost-effective.
It agreed the policy after a year-long consultation, in which evidence about the role earlier treatment can play in reducing transmission of the virus was considered.
The WHO’s HIV/AIDS director, Dr. Gottfried Hirnschall, said: “It will be very difficult to end AIDS without a vaccine – but these new guidelines will take us a long way in reducing deaths.
“We’re recommending earlier treatment – and also safer, simpler medicines that are already widely available.
“We also want to see better monitoring of patients, so they can see how well they’re doing on the treatment.
“This is not only about keeping people healthy and alive – the anti-retroviral drugs block transmission, so there is the potential for a major impact in preventing epidemics within different countries.”
Five companies make the daily combination pill, which can cost about $127 for a year’s individual treatment in countries where price reductions have been negotiated.
The WHO says there is an “encouraging trend” of countries using their own finances to fight the HIV/AIDS epidemic such as Zimbabwe, which has successfully used a levy on mobile phones.
The new recommendations also include providing drugs to all children under five with the virus, all HIV-positive pregnant and breastfeeding women and to people whose partner is uninfected.
In all of these cases, treatment would start regardless of how far the condition has damaged their immune system.
Dr. Gottfried Hirnschall added: “We are still seeing young children lagging behind in terms of access to treatment. Two-thirds of adults that need anti-retroviral drugs get them, but only a third of young children.”
The Global Fund – set up to fight Aids, tuberculosis and malaria – welcomed the guidelines as “very timely”.
Its executive director, Dr. Mark Dybul, said: “This is an example of how the Global Fund and the WHO work together to support countries as we move towards removing HIV as a threat to public health.”
MSF (Medecins Sans Frontieres / Doctors Without Borders) warned extra political and financial support would be needed for implementing the recommendations, which it said were “ambitious but feasible”.
MSF medical co-ordinator in South Africa Dr. Gilles van Cutsem said: “With these new guidelines our collective goal should now be to scale up without messing up: to reach more people, retain them on treatment, and with an undetectable viral load.
“There’s no greater motivating factor for people to stick to their HIV treatment than knowing the virus is <<undetectable>> in their blood.”
The UN’s latest assessment of global cases of HIV/AIDS shows there has been a further drop in new infections among children.
There were 330,000 new infections in children last year – the figure is 24% lower than in 2009.
But the report by UNAids also warns “significant additional effort is required” if broader targets to tackle HIV/Aids are to be achieved by 2015.
The agency’s director said the pace of progress was speeding up.
Overall, 34 million people around the world are now thought to have the virus that causes Aids.
The number of new infections in adults has stayed broadly stable for the past four years – at about 2.5 million new cases a year.
Many more people with HIV now receive life-saving drugs which keep the virus under control. But the report estimates seven million people who need treatment still do not have it.
The UN’s latest assessment of global cases of HIV-AIDS shows there has been a further drop in new infections among children
Sub-Saharan Africa remains the most severely affected part of the world, though some countries there have made impressive efforts in reducing fresh cases.
The executive director of UNAids, Michel Sidibe, said: “Ethiopia, Malawi and Botswana have achieved big reductions in new infections, showing they are capable of controlling the epidemic.
“Twenty-five countries have reduced the number of new infections by more than 50%.
“In general, we’ve moved from a phase of political rhetoric to programmes being implemented and having an effect.
“But some countries aren’t using the right strategies – Russia, for example, where infections are still growing.”
The report also shows significant increases in AIDS-related deaths in Eastern Europe, Central Asia, the Middle East and North Africa.
UNAids is monitoring progress against targets such as reducing sexual transmission of HIV by 50% by 2015, and providing antiretroviral therapy to all 15 million people who need it.
The report shows where challenges remain. For example, it says there needs to be a scaling up of efforts to offer circumcision to men, which trials have shown is effective in preventing some new infections.
And it describes how “intensive efforts” are under way to find effective non-surgical approaches to circumcision – avoiding the need for scalpels or stitches – so that trained nurses could carry out the procedure rather than doctors.
Dr. Manica Balasegaram, Executive Director of Médecins Sans Frontières/Doctors Without Borders Access Campaign, said: “Scaling up HIV treatment to 15 million people from 8 million today is feasible and has the crucial triple benefit of reducing illness, reducing death, and reducing the risk of transmission.”
Experts writing in the Lancet have explained that drug-resistant HIV has been increasing in parts of sub-Saharan Africa over the last decade.
Studies on 26,000 untreated HIV-positive people in developing countries were reviewed by the team.
They said resistance could build up if people fail to stick to drug regimes, and because monitoring could be poor.
The researchers, from the World Health Organization (WHO) and University College London (UCL) found the most rapid increase in drug resistance occurred in East Africa, at 29% per year. In Southern Africa, it was 14% per year.

Drug-resistant HIV has been increasing in parts of sub-Saharan Africa over the last decade
There was no change in resistance over time in Latin America and in West and Central Africa.
Writing in the Lancet, authors Dr. Silvia Bertagnolio from the WHO and Dr. Ravindra Gupta at UCL said: “Without continued and increased national and international efforts, rising HIV drug resistance could jeopardize a decade-long trend of decreasing HIV/Aids-related illness and death in low- and middle-income countries.”
Dr. Ravindra Gupta said: “Drug resistance is a consequence of people not taking their medication properly.
“We do expect to see drug resistance, and it’s at around 10% in the UK and US. But here, we monitor people regularly and switch people to different drugs if they develop resistance.”
He said that quite basic measures could help people to better adhere to drug regimes in developing countries, such as access to food and clean water so they can take their drugs, and monitoring patients as effectively as possible.
The researchers said no changes were needed to the drug regimes, but Dr. Ravindra Gupta said: “This work gives us an early-warning that things could get worse.”
OraQuick home HIV test is expected to go on sale in the US within months, after winning regulator approval.
The OraQuick test checks saliva from a mouth swab for HIV and can produce results in 20 to 40 minutes.
Government estimates suggest 1.2 million people in the US are HIV-positive, but 20% do not know they are.
The Food and Drug Administration (FDA) has said it hopes the over-the-counter test will reach people who might not otherwise get tested.
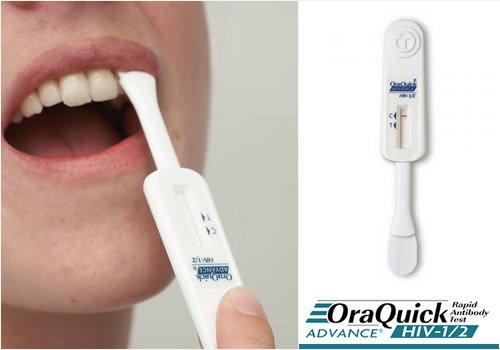
OraQuick home HIV test is expected to go on sale in the US within months, after winning regulator approval
The test is expected to be sold in as many as 30,000 pharmacies and homeware shops, as well as online.
The manufacturer, OraSure, has not said how much the test will sell for but confirmed it would cost less than $60.
“We expect all the major retail outlets to carry this product,” chief executive Douglas Michels said.
The company is planning a “pretty massive effort” to market the test, Douglas Michels added.
Approval of the test has been praised by HIV/AIDS awareness groups.
“This test will allow anyone to empower themselves to know their HIV status when, how and with whom they want to,” Tom Donohue, founding director of Who’s Positive, told the Associated Press.
But the FDA has highlighted in its announcement that the test may not be 100% accurate, and has stressed the need for additional testing by medical professionals to confirm the result.
OraSure said that in trials the test was able to correctly detect HIV among people carrying the virus only 92% of the time.
It was 99% accurate for negative results – or identifying that someone does not carrying the virus.
Meanwhile, Dr Jonathan Mermin, director of the HIV unit at the Centers for Disease Control and Prevention, said people who receive negative results should take the test again after three months, because it can take weeks before antibodies to HIV appear.
Healthcare professionals have been using a version of the OraQuick test since 2002.
For the last two decades there have been about 50,000 new cases of HIV in the US each year.
US Antiviral Drugs Advisory Committee, which advises the FDA, has for the first time backed a drug to prevent HIV infection in healthy people.
The panel of health experts recommended US regulators approve daily pill Truvada for use by people considered at high risk of contracting the AIDS virus.
The US Food and Drug Administration (FDA) is not required to follow the panel’s advice, but it usually does.
Some health workers and groups active in the HIV community have opposed the approval of the drug.
However, the move could prove to be a new milestone in the fight against HIV/AIDS.
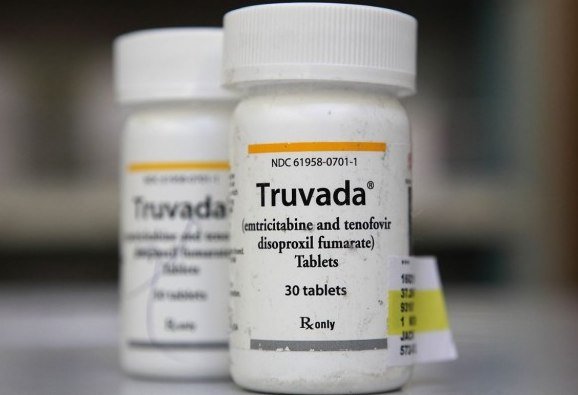
The Antiviral Drugs Advisory Committee recommended US regulators approve Truvada for use by people considered at high risk of contracting the AIDS virus
Truvada is already approved by the FDA for people who are HIV-positive, and is taken along with existing anti-retroviral drugs.
Studies from 2010 showed that Truvada, made by California-based Gilead Sciences, reduced the risk of HIV in healthy gay men – and among HIV-negative heterosexual partners of people who are HIV-positive – by between 44% and 73%.
The Antiviral Drugs Advisory Committee voted 19-3 in favor of prescribing the drug to the highest risk group – non-infected men who have sex with multiple male partners.
They also approved it, by majority votes, for uninfected people with HIV-positive partners and for other groups considered at risk of acquiring HIV through sexual activity.
The votes followed an 11-hour meeting of the panel in Silver Spring, Maryland, and a lengthy public comments session.
Opposition to the prospect of approving the drug is based on concerns that users could gain a false sense of security, and fears of a drug-resistant strain of HIV.
There is also concern that the high cost of Truvada could divert limited funding from more cost-effective options.
“We need to slow down. I care too much about my community not to speak my concerns,” said Joey Terrill, of the AIDS Healthcare Foundation, which campaigned against the drug’s approval.
Nurse Karen Haughey told the panel: “Truvada needs to be taken every day, 100% of the time, and my experience as a registered nurse tells me that won’t happen.
“In my eight years, not one patient that I’ve cared for has been 100% adherent.”
But others welcomed the panel’s recommendation.
“This brings us closer to a watershed for global HIV prevention efforts,” said Mitchell Warren, executive director of the AIDS Vaccine Advocacy Coalition, after the vote.
The FDA is expected to make its decision by 15 June.





