Diclegis: Kim Kardashian Compensated by Duchesnay for Promoting Morning Sickness Drug
The FDA has condemned Kim Kardashian’s promotion of morning sickness prescription drug Diclegis on social media.
Kim Kardashian, who is 5-month pregnant with her second child, posted a selfie last month holding up a branded bottle of the pills alongside text endorsing their effects.
Diclegis maker, Canada-based Duchesnay, later confirmed it had compensated Kim Kardashian for “sharing her experience”.
However, the FDA has attacked the posts for failing to flag potential side effects.
The agency has ordered the drugmaker to stop promoting its product in this manner.
Kim Kardashian’s posts talked of her own use of the drug Diclegis, before stating: “Most importantly, it’s been studied and there was no increased risk to the baby.”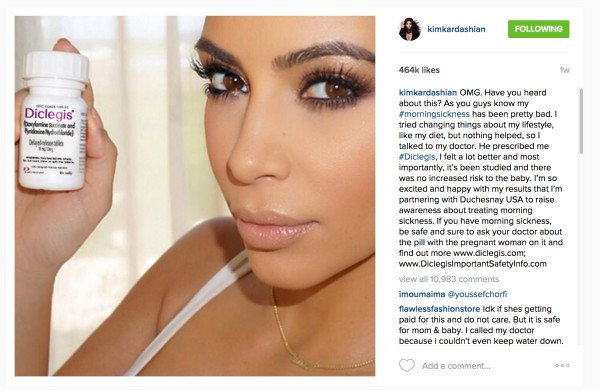
The 34-year-old reality star provided a link to the pharmaceutical company’s own realted safety warnings, but did describe the potential risks herself.
One side effect of a normal dose of Diclegis is drowsiness. An overdose can cause vertigo, mental confusion and an abnormal heart rate.
“The social media post is misleading because it presents various efficacy claims for Diclegis, but fails to communicate any risk information,” FDA division director Robert Dean wrote in a complaint to Duchesnay.
“Because the violations described above are serious and repeated, we request, further, that your submission includes a comprehensive plan of action to disseminate truthful, non-misleading, and complete corrective messages about the issues discussed in this letter to the audiences that received the violative promotional materials.”
Robert Dean added that the correction should be distributed “using the same media” as the original messages, indicating that the warnings should appear on Kim Kardashian’s social media accounts.
Kim Kardashian’s selfie has since been deleted from Instagram and Facebook – although a tweet alluding to the posts remains online.
Duchesnay said that it would issue a formal response ahead of a August 21 deadline.
