Pancreatic cancer: new clinical trials have started
BioCancell has begun enrollment of phase IIb BC-819 pancreatic cancer clinical trial, while Halozyme has started randomized, controlled clinical trial with PEGPH20 in patients with advanced pancreatic cancer.
Tikcro Technologies Ltd. announced on October 6 that BioCancell, a clinical stage biotechnology company focused on developing targeted cancer therapies for solid cancer tumors, has commenced enrollment of its Phase IIb pancreatic cancer clinical trial with a first treatment of the first patient.
The FDA granted BioCancell a Fast Track designation for BC-819, as a treatment for locally-advanced pancreatic cancer. Fast Track designation is expected to expedite FDA review of BC-819 and the process of clinical trials.
The trial will examine the effect of BioCancell‘s BC-819 in sequence with Gemzar (gemcitabine), the current standard chemotherapy for pancreatic cancer, in patients with advanced, unresectable (inoperable) pancreatic cancer.
The clinical trial is expected to include approximately 100 participants in multiple medical centers in Europe, Israel and the U.S. Half of the participants will be treated with the combination of BioCancell‘s BC-819 and gemcitabine, and the other half will be treated with gemcitabine alone.
The primary endpoint of the trial is progression-free survival. Secondary objectives include overall survival, response rate and resectability of the target tumor lesion.
An interim analysis is planned for after the recruitment of the first 18 patients in order to determine the appropriate dose of BC-819 to utilize for the remainder of the trial.
Tikcro (Tel Aviv, Israel) has holdings in BioCancell Therapeutics, Inc. (Jerusalem, Israel), a clinical-stage biopharmaceutical company operating in the area of cancer treatment.
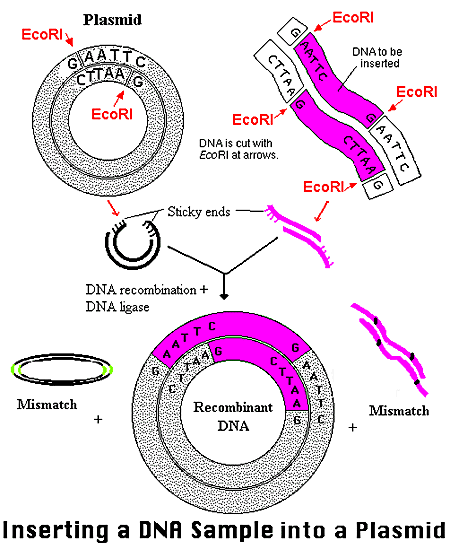
BC-819, used to treat pancreatic cancer, is a double stranded DNA plasmid construct that incorporates the gene for diphtheria toxin (DTA) under the regulation of the promoter sequence for H19 gene.
BC-819 is a double stranded DNA plasmid construct that incorporates the gene for diphtheria toxin (DTA) under the regulation of the promoter sequence for H19 gene.
BC-819 uses the H19 gene to target susceptible tumor cells. A vehicle is constructed to gain access to the targeted cell, and the host cell’s internal machinery is directed to produce Diptheria Toxin (DTA). BC-819 enters all dividing cells, but while it will destroy the cancerous cells that express H19 it will be just a strand of non-functional DNA in non-cancerous cells.
DTA is one of the most lethal toxins known to mankind, and has proved to be the most efficient agent for the targeted destruction of cancerous growths. Because of this, it has been a frequently-used component of targeted approaches to cancer therapy, including immunotoxins and gene therapies, and has been approved by the FDA for a number of cancer treatments. There are two strong safety factors protecting the patient receiving DTA. The first is that Western societies are fully immunized against Diphtheria Toxin. The second is that the technology only utilizes the A fragment of the toxin, not the B fragment that would allow the toxin to replicate itself.
BioCancell is conducting a phase IIb clinical trial for the treatment of pancreatic cancer, phase IIb clinical trial for the treatment of superficial bladder carcinoma cancer and a Phase I/IIa clinical trial for the treatment of ovarian cancer.
Halozyme has begun randomized, controlled phase 2 clinical trial to assess safety, tolerability and efficacy of gemcitabine combined with PEGPH20 in patients with advanced pancreatic cancer.
Halozyme Therapeutics, Inc., a biopharmaceutical company developing and commercializing products targeting the extracellular matrix for the diabetes, cancer, dermatology and drug delivery markets, announced on October 5 the commencement of patient dosing in a Phase 2 clinical trial with pegylated rHuPH20 (PEGPH20) in patients with stage IV previously untreated pancreatic cancer. This multi-center, international, randomized, placebo-controlled trial will study the safety, tolerability and efficacy of gemcitabine plus PEGPH20 compared to gemcitabine plus placebo.
“Pancreatic cancer continues to be an extremely challenging disease for both patients and physicians alike and, unfortunately, most patients with pancreas cancer live less than one year from the time of diagnosis. However, a number of recent observations from both preclinical and early Phase 1 clinical studies suggest that PEGPH20 may render pancreatic cancers more susceptible to conventional chemotherapy by depleting the tumor stroma of hyaluronan, improving vascular perfusion and preferentially increasing drug delivery to the tumor bed,” said Sunil R. Hingorani of the Fred Hutchinson Cancer Research Center, a pancreatic cancer biologist and principal investigator for the trial.
The trial, which includes a single arm run-in stage, will evaluate gemcitabine plus PEGPH20 compared to gemcitabine plus placebo in patients with previously untreated metastatic pancreatic ductal adenocarcinoma. In the Phase 2 portion, patients will be randomized to receive either PEGPH20 plus gemcitabine or placebo plus gemcitabine. Over 140 patients are expected to be enrolled globally.
The primary endpoint of the Phase 2 portion of the trial is overall survival (OS). The trial will also evaluate secondary endpoints such as CA19-9, objective response rate and duration of response. CA19-9 is a tumor marker tested from blood samples in patients with pancreatic cancer.
Certain solid tumor types (colon, breast, pancreatic and prostate cancers) have been shown to accumulate high levels of hyaluronic acid (hyaluronan) which creates a protective network surrounding the tumor cell.
PEGPH20 is a PEGylated form of rHuPH20 modified to allow it to survive in the blood stream. PEGPH20 degrades hyaluronan (HA) which is a gel-like component found in tissues throughout the body. Certain solid tumor types (colon, breast, prostate, pancreatic cancer) have been shown to accumulate high levels of HA which creates a protective network surrounding the tumor cell. By degrading the HA, PEGPH20 may increase the accessibility of tumor cells to chemotherapeutic agents and improve the effectiveness of the treatment.
Halozyme Therapeutics (San Diego, California) is a biopharmaceutical company developing and commercializing products targeting the extracellular matrix for the insulin, cancer, dermatology and drug delivery markets. The company’s product portfolio is based primarily on intellectual property covering the family of human enzymes known as hyaluronidases and additional enzymes that affect the extracellular matrix. Halozyme‘s Enhanze(TM) technology is a novel drug delivery platform designed to increase the absorption and dispersion of biologics. The company has key partnerships with Roche, Baxter, ViroPharma and Intrexon to apply Enhanze technology to therapeutic biologics including Herceptin®, MabThera®, immunoglobulin, Cinryze® and recombinant human alpha 1-antitrypsin. Halozyme‘s Ultrafast Insulin program combines its rHuPH20 enzyme with mealtime insulins, which may produce more rapid absorption, faster action, and improved glycemic control. The product candidates in Halozyme‘s pipeline target multiple areas of significant unmet medical need.
Pancreatic cancer is the fourth leading cause of cancer-related death in the world.
The U.S. National Cancer Institute estimates that in 2011 there will be more than 44,030 new cases of pancreatic cancer and 37,660 deaths from the disease.
Pancreatic cancer is a malignant tumor of the pancreas. The most common type of pancreatic cancer, accounting for 95% of these tumors is adenocarcinoma, which develops within the exocrine component of the pancreas. A minority arises from the islet cells and is classified as a neuroendocrine tumor.
Sometimes called a silent killer (because early pancreatic cancer often does not cause symptoms), pancreatic cancer’s symptoms may include: upper abdominal pain, appetite loss, nausea, vomiting, rapid weight loss, jaundice, itching, diabetes mellitus (or high blood sugar levels), Trousseau sign, depression.
Lots of patients with pancreatic cancer develop diabetes months to years before they are diagnosed with pancreatic cancer, suggesting new onset diabetes in a senior may be an early sign of pancreatic cancer.
Pancreatic cancer first metastasizes to regional lymph nodes, generally, and later to the liver and, less commonly, to the lungs; occasionally to bone or brain.
Risk factors for pancreatic cancer may include: heredity (family history of pancreatic cancer or pancreatitis), age, smoking, diets low in vegetables and fruit, high in red meat and high in sugar-sweetened drinks (fructose has been linked to growth of pancreatic cancer cells), obesity, diabetes, chronic pancreatitis, Helicobacter pylori infection, gingivitis or periodontal disease.
